FDA accepts Drug Master File, signifying a new milestone for Touchlight’s enzymatic doggybone DNA technology
Hampton, UK, 24 January 2023 – Touchlight, a biotechnology company pioneering enzymatic DNA production to enable genetic medicines, today announced a ground-breaking new milestone for its enzymatic doggybone DNA (dbDNA™), following the FDA acceptance of the Drug Master File (DMF) for GMP grade dbDNA.
- Drug Master File simplifies the U.S. regulatory process for utilising Touchlight’s doggybone DNA and accelerates regulatory filings.
- doggybone DNA is the first enzymatic DNA platform with a Drug Master File, representing a significant step towards regulatory adoption.
This achievement marks the first time a DMF has been accepted for an enzymatically produced DNA platform, representing a sign of the growing interest and adoption of enzymatic DNA across the genetic medicine industry.
The FDA acceptance of the DMF for GMP grade dbDNA will ensure that customers no longer need to prepare a standalone section in their fillings when dbDNA is used as a starting material or intermediate, saving considerable time and effort of regulatory and QA functions.
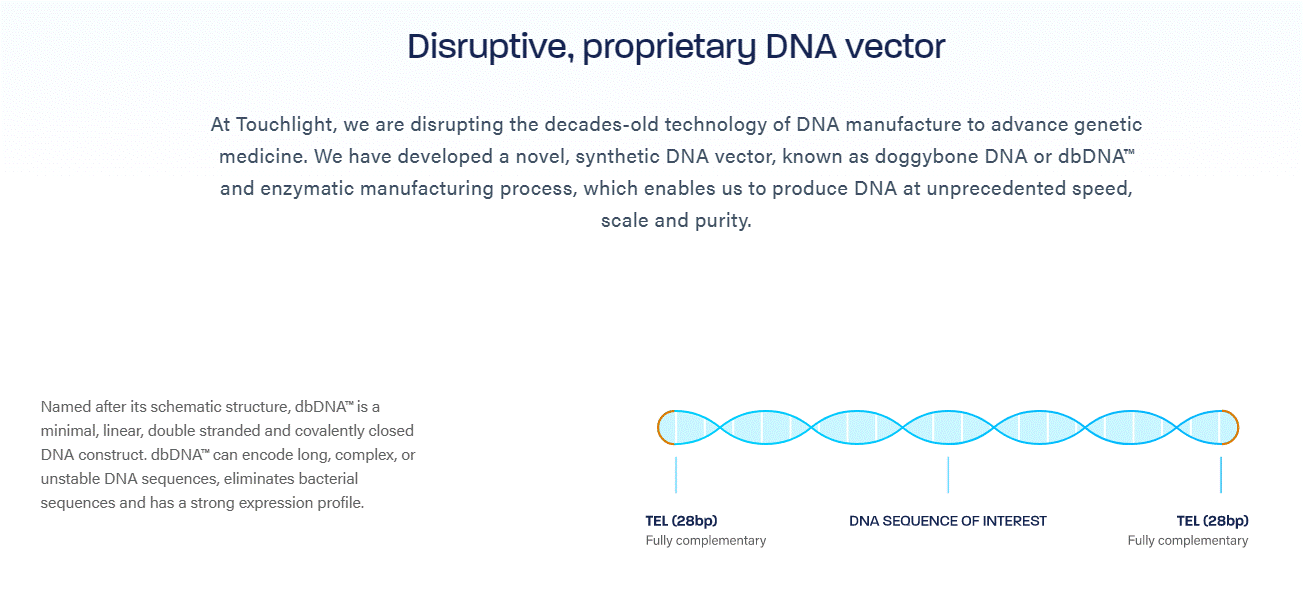
Touchlight’s dbDNA is a minimal, linear, double stranded, covalently closed DNA vector which is produced through an enzymatic manufacturing process as an alternative to bacterial derived plasmid DNA. dbDNA can accommodate genes of interest of up to at least 20kb and is linearly scalable, making it highly adaptable to support a range of genetic medicines and is ideal for the development of mRNA vaccines, therapeutics, viral vector-based gene therapies and beyond. Touchlight’s doggybone DNA technology was utilised for the first time in human clinical studies in 2022, with multiple further studies initiating in 2023.
Touchlight’s CDMO customers now have the option to gain access to the dbDNA proprietary manufacturing process information through the DMF for their IND submissions in the U.S. This is a direct result of Touchlight’s strategy for regulatory engagement and effort to enable broad utilisation of dbDNA across the genetic medicine industry.
Satish Muchakayala, Director of Regulatory Affairs, Touchlight commented “Achieving FDA acceptance of our DMF is a win-win for Touchlight’s customers and regulators, avoiding duplication of effort at all levels and thereby helping accelerate regulatory submission and approvals.”





