Defined stem cell media offers easy transition to clinical manufacturing
AMSBIO has launched GMP grade StemFit® for Differentiation – a chemically defined and animal component-free formulation that enables unmatched differentiation of human Induced Pluripotent Stem (hiPS) and Embryonic Stem (hES) cells. This medium now complies with Good Manufacturing Practices, as required for downstream clinical applications.
The unique chemically defined composition of StemFit® for Differentiation minimizes lot-to-lot variation, enabling highly consistent cell differentiation. The animal-origin free composition minimizes the risk of immunogenic contamination. Manufactured to strict GMP guidelines, the new gold standard version of StemFit® for Differentiation offers highly reproducible expansion and differentiation of human pluripotent stem cell-derived cells and tissues in clinical manufacturing applications.
Dr. Satoshi Okamoto from the Yokohama City University Graduate School of Medicine said “Using StemFit® Basic03 for iPSC cell culture with StemFit® for Differentiation for differentiation, we were able to adopt animal-origin free reagents, which made our culture protocol very stable.”
By the end of 2025, all StemFit® formulations including Basic03, Basic04 CT, and key proteins including bFGF and Activin A, will be available exclusively as GMP-grade formulations. Transitioning the range to GMP grade ensures that every lab using StemFit® can seamlessly move from research to clinical applications without needing to retest materials or change protocols and so reducing complexity and cost.
To keep GMP grade formulations adoption accessible, manufacturing processes have been optimized to minimize the cost difference between research and GMP-grade versions. This ensures that labs can upgrade to GMP without significant financial burden.
Aisha Amari, a stem cell specialist at AMSBIO commented “StemFit® has earned the trust of researchers and biotech companies worldwide, with nearly 1,500 citations and growing adoption in cell and gene therapy”.
To learn more about GMP compliant products and services from AMSBIO please visit https://www.amsbio.com/gmp/gmp-products-services/. For further information about StemFit® for Differentiation please visit https://www.amsbio.com/stemfit-for-differentiation/ or contact AMSBIO on +31-72-8080244 / +44-1235-828200 / +1-617-945-5033 / info@amsbio.com.
Now part of the Europa Biosite group of companies, AMS Biotechnology (AMSBIO) is recognized as a leading transatlantic company contributing to the acceleration of discovery through the provision of cutting-edge life science technology, products, and services for R&D in the medical, nutrition, cosmetics, and energy industries. AMSBIO has in-depth expertise in extracellular matrices to provide elegant solutions for studying cell motility, migration, invasion, and proliferation. This expertise in cell culture and the ECM allows AMSBIO to partner with clients in tailoring cell systems to enhance organoid and spheroid screening outcomes using a variety of 3D culture systems, including organ-on-a-chip microfluidics. For drug discovery research, AMSBIO offers assays, recombinant proteins, and cell lines. Drawing upon a huge and comprehensive biorepository, AMSBIO is widely recognized as a leading provider of high-quality tissue specimens (including custom procurement) from both human and animal tissues. The company provides unique clinical grade products for stem cells and cell therapy applications. This includes GMP cryopreservation technology, and high-quality solutions for viral delivery.
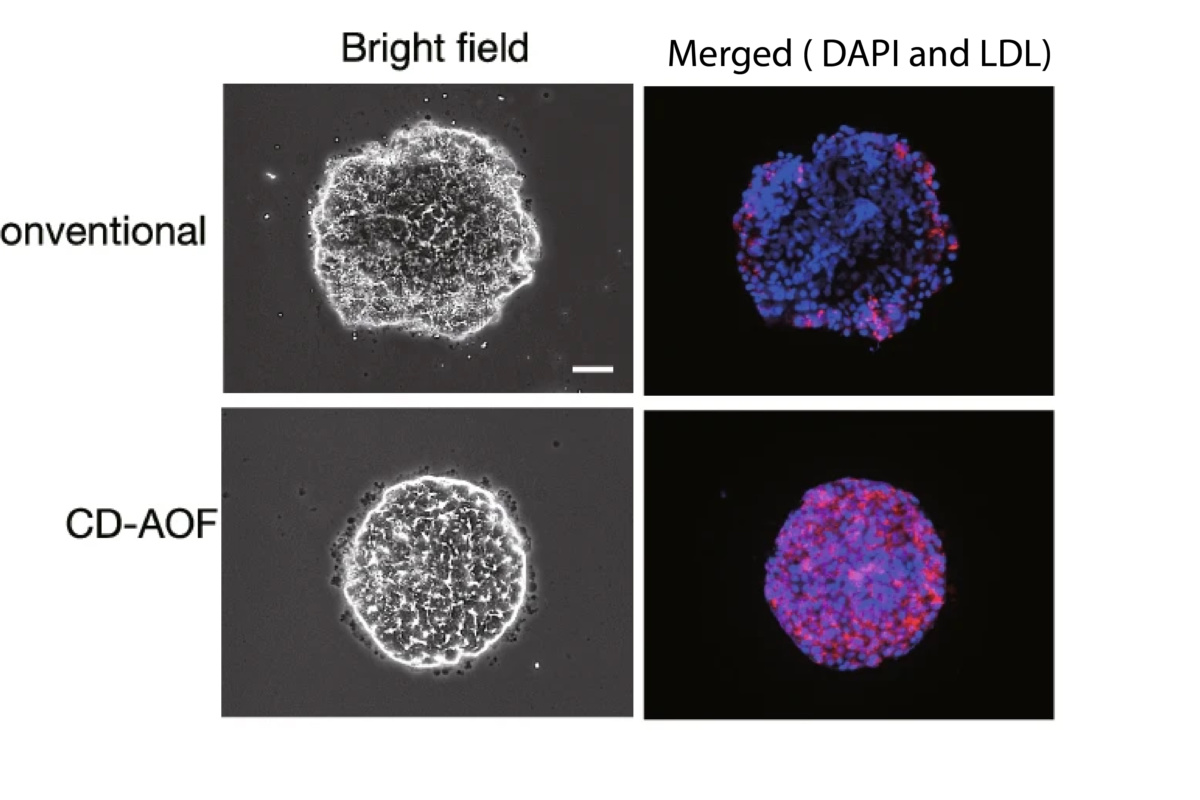
Image captions: A: StemFit® for Differentiation in use: Lipid incorporation study in all iPSC-LBs by using DiI-Ac-LDL (Acetylated Low-Density Lipoprotein labeled with 1,1’-dioctadecyl-3,3,3’,3’- tetramethylindo-carbocyanine perchlorate). Scale bar, 50 µm. Figure adapted from Sekine, K., Ogawa, S., Tsuzuki, S., Kobayashi, T., Ikeda, K., Nakanishi, N., Takeuchi, K., Kanai, E., Otake, Y., Okamoto, S., Kobayashi, T., Takebe, T., & Taniguchi, H. (2020). Generation of human induced pluripotent stem cell-derived liver buds with chemically defined and animal origin-free media. Scientific Reports, 10(1). https://doi.org/10.1038/s41598-020-73908-1