Cutting edge technology for vascularized kidney organoids
Abingdon, UK – AMSBIO has published an interview with Professor Ryuji Morizane, a renowned expert in renal research at Harvard Medical School. In his award-winning research*, Professor Morizane has successfully combined organoid and bioengineering technology to create kidney organoids that incorporate a vascular structure.
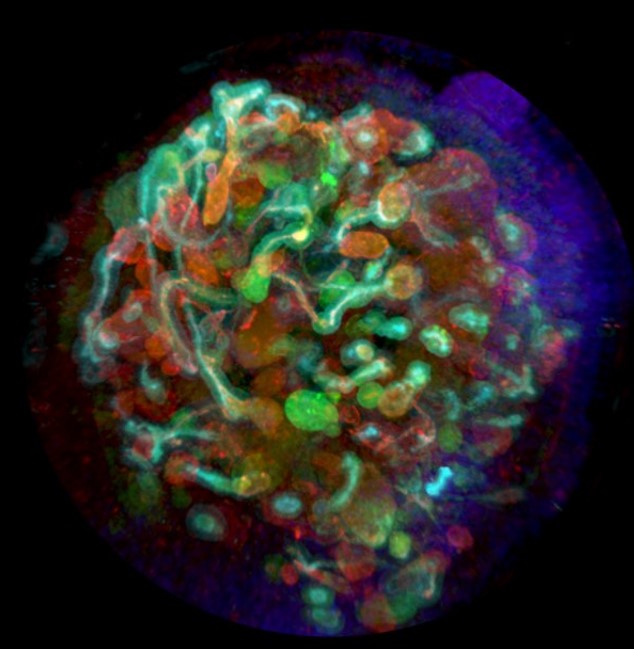
To help facilitate this pioneering work, Professor Morizane research group uses StemFit® – a feeder-free, chemically defined culture medium for embryonic (ES) and induced pluripotent stem cells (iPSC) which are without human or animal-derived components.
The kidney is a vital organ, which continuously filters the blood and maintains overall fluid homeostasis of the body. Due to the complexity of the structure of the kidney and its vascular networks, renal function is difficult to investigate in vitro, even with advanced 3D cell models. Recent developments in stem cell-based methods for producing small cell-culture models of organs (known as “organoids”) have accelerated kidney research considerably. But, until recently, most organoids have had almost no vasculature, which is critical to normal renal function.
In discussion about his research – Professor Morizane commented “There were many challenges we had to overcome to reliably produce vascularized renal organoids. In the past, we used to culture hPSCs with feeder cells, however frequent passaging and the removal process of feeder cells often lead to variations in cell quality. Using StemFit® culture medium, has allowed us to eliminate the use of feeder-culture, meaning we can produce a larger stock of iPSCs. This, combined with frequent checking of progenitor cell differentiation efficacy, has helped our researchers reduce batch-to-batch variation. Switching to StemFit® has also improved cell viability and growth, enabling us to improve gene-editing efficacy, and the production of more cells for experimentation”.
He added ”Our renal organoid technology shows great promise for use in disease modelling and toxicity testing. In the future, I would like to focus on translational research such as drug screening using kidney organoids – and also consider clinical applications. Here the challenge is to produce high-quality cells used for transplantation. For these new applications it is necessary that we develop cost-efficient culture methods to produce iPSCs and the differentiation protocols of organoids.”
The StemFit® range of xeno-free, chemically defined media from AMSBIO is proven to effectively maintain induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) under feeder-free conditions during the reprogramming, expansion and differentiation phases of stem cell culture. Available in research and GMP grade formulations, StemFit® is the market leader for colony-forming efficiency – combining this with lower than standard media volume consumption to offer the most cost-effective colony expansion compared to leading competitors. StemFit® is part of AMSBIO’s portfolio of Stem Cell Synergy Solution products for streamlining and improving the efficiency of ES/iPS Cell Culture for basic through to clinical research.
To read the interview in full please visit https://www.amsbio.com/news/cutting-edge-technology-for-vascularized-kidney-organoids/. For further information on the new StemFit® please visit https://www.amsbio.com/stem-cell-synergy/






